Providing integrated prescription validation services
Pharmacy applications enabled for Electronic Prescription of Controlled Substances (EPCS) must meet strict U.S. Drug Enforcement Administration (DEA) requirements.
Applications must be verified by a third-party auditor to follow a DEA mandated process, which includes the verification of digital signatures and certificate validations. These validations must be performed in compliance with National Institute of Standards and Technologies (NIST) requirements.
IdenTrust solutions fully meet identity-specific DEA requirements for pharmacy applications and IdenTrust cross-certified Device Certificates are ideal for digitally signing incoming EPCS messages. Digital signatures created with IdenTrust certificates can be fully relied upon by U.S. Federal agencies.
Simplifies and accelerates application development

Ensures DEA compliance and is trusted by auditors

Supports NCPDP SCRIPT and Surescript® message formats

Leverages IdenTrust EPCS knowledge and digital signing expertise
IdenTrust Pharmacy Solutions
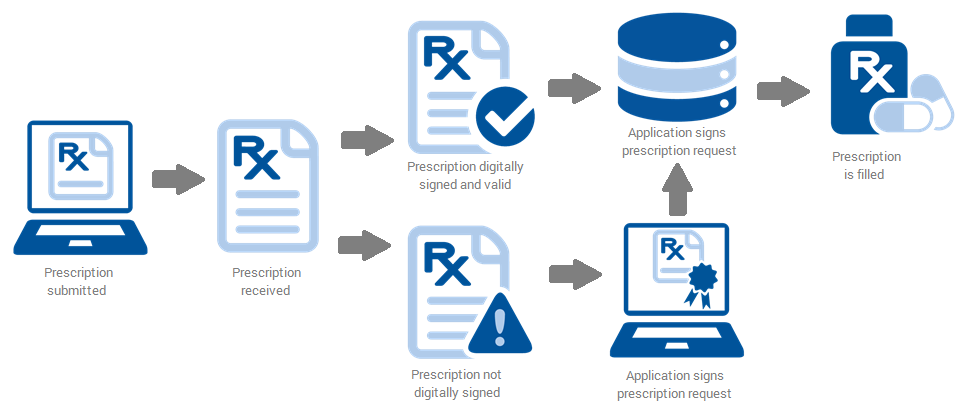
Upon receipt of a digitally signed EPCS message, the digital signature and associated certificate must be validated by the pharmacy.
Contact IGCsales@IdenTrust.com for a complimentary web meeting to learn about DEA EPCS requirements for pharmacy applications and other IdenTrust healthcare compliance solutions.
Mechanics of Message Validation
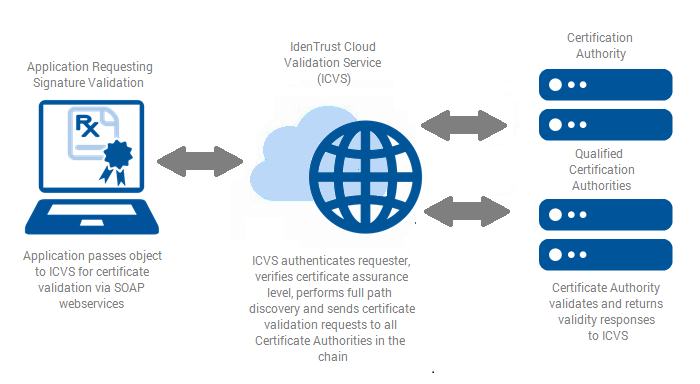
The IdenTrust Cloud Validation Service (ICVS) provides pharmacy applications with a simple cloud-based service that performs digital signature and certificate validations in a manner that meets DEA and NIST requirements. Validations are accomplished with a simple web services call.
IdenTrust Cloud Validation Service (ICVS)
Contact IGCsales@IdenTrust.com for a complimentary web meeting to learn about DEA EPCS requirements for pharmacy applications and other IdenTrust healthcare compliance solutions.
